Introduction
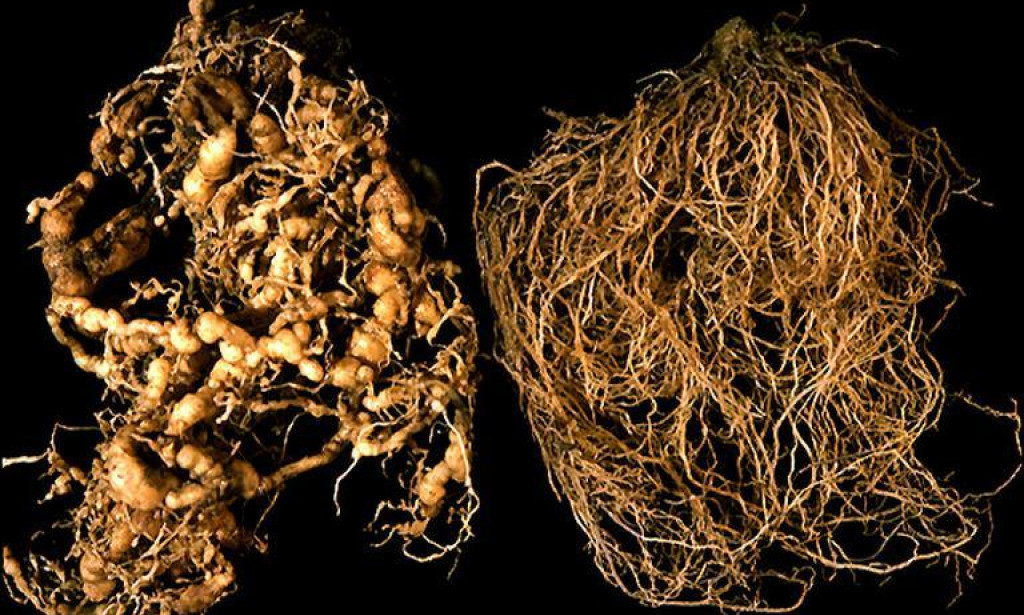
Root‐knot nematodes (RKN), (Meloidogyne spp.) are obligate, sedentary endo-parasites of more than 3000 species of plants. The root-knot nematodes (genus Meloidogyne) are among the world’s most destructive crop pests, causing global agricultural losses close to $80 billion annually (Moens, et al., 2009). The genus Meloidogyne contains over 90 described species and each of these species typically has an extremely broad host range as many as 3000 plant species (Trudgill and Blok, 2001). During parasitism, RKNs engage in prolonged and intimate relationships with their host plants (up to six weeks) often involving complex morphological and physiological alterations of host cell into specialized cell called giants (Figure:1). The giant cells are essential for successful parasitism because this unique feeding structure provides nutrition to develop second stage juvenile (J2) in to adult (Abed, et al., 2003).
Controlling of obligate plant parasitic-root-knot nematode is generally difficult and also with the banning of nematicides chemicals that control nematode, because of adverse environmental impacts, it is imperative that new safe disease control strategies for nematode management should be developed and implemented. In order to develop new and safe disease control strategies, it is essential to understand the molecular, cellular and genetic mechanisms of how RKN interact with diverse host environments. Considerable variation in ability to break crop resistance and to reproduce on different crop species is observed both between and within species (Williamson and Roberts, 2009). The main feature of RKN is its potential host range encompassing more than 3,000 plant species. The adaptation of RKN species to its environment raises questions about genome plasticity leading to genetic variation and adaptive evolution. It has been proposed that epigenetic as well as cytogenetic variation mechanisms might in part be responsible for the generation of phenotypic variants that provide material for rapid adaptation of more than 3000 plant host environments (Trudgill and Blok, 2001).Thus, RKN species constitutes a unique model system to study the links between variation in genome structure, mode of reproduction, and adaptation to environment and host, in relation with parasitic success. Hence, in this review paper, therefore, we have conducted extensive literature reviews to identify the cytogenetic status (from classical cytogenetic to molecular cytogenetics) of root‐knot nematodes (Meloidogyne spp.). Specifically, the status of the studied areas of cytogenetic in RKN as well as the pattern of cytogenetic variation and evolution were summarized.
In conclution, Root-knot nematodes (RKN), Meloidogyne spp., belong to the order Tylenchida. RKN have undergone extensive cytogenetic diversification in chromosome number and mode of reproduction. They exhibit a wide continuum of variation in their reproductive strategies, ranging from amphimixis to obligatory mitotic parthenogenesis. Majority of Meloidogyne speciesare apomictic. Some species can reproduce by automictic. Very few RKN species, M. carolinensis, M. megatyla, M. microtyla, M. pini are reproduced by amphimixis. Sex determination in the genus is not yet established. Environmental factors play a role in the sexual differentiation and inter-sexes in the genus. The trend from amphimictic reproduction to apomixis is generally associated with shorter life cycles, higher reproductive rates and increasing pathogens. The association between mode of reproduction and ploidy level were observed in RKN species. For, example, obligatory cross-fertilization occurs in some diploid and polyploidy, whereas automixis and prevail in most polyploid and aneuploid forms.
RKN are also highly variable with respect to their chromosomal complement, even populations of the same parthenogenetic species may have different number of chromosomes. The basic chromosome number of the genus is n = 18. Chromosome size of RKN ranged from 0.4 - 1.6 um. Although some variation in chromosome size within the chromosomal complement of, each species exists, differences are not very extensive. Extensive variation in chromosome number and mode of reproduction are important in cytotaxonomy. It has been proposed that Meloidogyne karyotype evolved from Heterodera karyotype by polyploidizadon since the ratio of basic chromosome number of Heterodera (n=9) to Meloidogyne (n=18) are 1:2. Alternatively, RKN karyotype may have been derived from the Heterodera karyotype by chromosomal fragmentation since its chromosome is much smaller than Heterodera. Based on cytogenetic and isoenzyme data the following assumptions are currently widely accepted: (1) the ancestral RKN were amphimictic animals, and the rare amphimictic species encountered today (eg M. carolinensis, (M. megatyla) are considered as their closest relatives; (2) parthenogenetic species evolved from amphimictic species; (3) obligatory parthenogenetic (mitotic) species evolved from facultative parthenogenetic (meiotic) species, following suppression of meiosis during oocyte maturation. In conclusion, data reviewed in this paper show that karyotypes of Meloidogyne spp., are not properly established.


You must be logged in to post a comment.